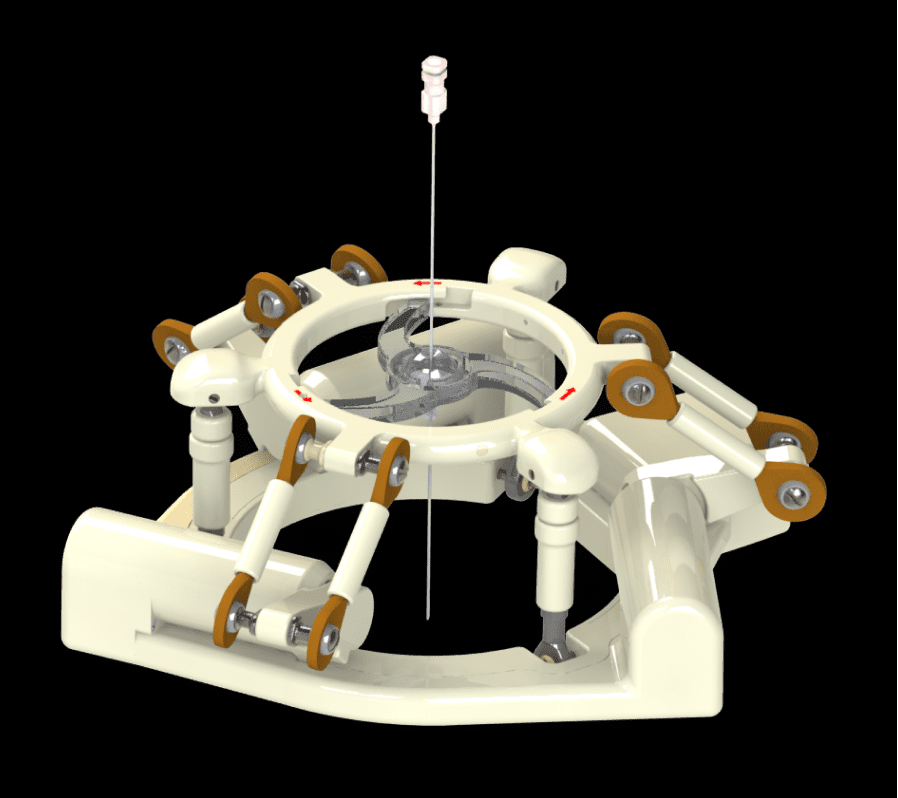
NDR Medical Technology's ANT-X Receives FDA Clearance, Enabling Image-Guided Surgeries
Singapore, Singapore, July 17, 2023 (VSNewsNetwork.com) - NDR Medical Technology has achieved a significant milestone as its ANT-X interventional robot receives FDA 510(k) clearance. ANT-X is the world's first automated robotic device designed to assist clinicians in precise needle placement for Percutaneous Nephrolithotomy (PCNL), a urology procedure for kidney stone removal, using image-guided fluoroscopy.
The FDA clearance of ANT-X marks a major advancement in the field of urology and brings new possibilities for improved treatment outcomes for patients with kidney stones. With the capability to enhance the success of PCNL procedures, urologists will be able to perform image-guided access with speed and precision, benefiting a larger number of patients. NDR Medical Technology aims to revolutionize the standard of care in healthcare and seeks partnerships and investors to continue developing breakthrough solutions.
"This milestone is a sign of consistent good traction made by the team as it continues to expand the applications of its technology," said Hsien-Hui Tong, Executive Director – Investments at SGInnovate. NDR Medical Technology's ANT-X paves the way for safer and more efficient image-guided surgeries, positioning the company as a leader in AI-empowered interventional robotics.
Learn more about NDR Medical Technology and the ANT-X robot at www.ndrmedical.com.
Source: NDR Medical Technology via PR.com